
Chemistry, 07.07.2019 15:00 gdominguez2005
H+ ions increase in concentration at lower ph values. calculate how many more h+ ions there are in a solution at a ph = 2 than in a solution at a ph = 6. find the concentration of h+ ions at a ph = 2 and at a ph = 6 in table b. then divide the concentration of h+ ions at a ph = 2 by the of h+ ions at a ph = 6. record your answer in table c. what is the concentration of h+ ions at a ph = 2? mol/l what is the concentration of h+ ions at a ph = 6? mol/l how many more h+ ions are there in a solution at a ph = 2 than in a solution at a ph = 6? need asap give brainliest

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

You know the right answer?
H+ ions increase in concentration at lower ph values. calculate how many more h+ ions there are in a...
Questions

Mathematics, 28.06.2019 16:30

Mathematics, 28.06.2019 16:30

English, 28.06.2019 16:30





History, 28.06.2019 16:30










Biology, 28.06.2019 16:30

Computers and Technology, 28.06.2019 16:30

![pH=-log[H⁺]](/tpl/images/0062/1602/22ab6.png)
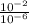 = 10⁴
= 10⁴


