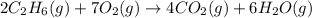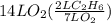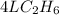Chemistry, 07.07.2019 15:00 villarrealc1987
2c2h6(g) + 7o2(g) → 4co2(g) + 6h2o(g) in the equation, if 14 l of ethane (c2h6) and 14 l of oxygen (o2) combined and burned to completion, which gas will be leftover after the reaction, and what is the volume of that gas remaining

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1
You know the right answer?
2c2h6(g) + 7o2(g) → 4co2(g) + 6h2o(g) in the equation, if 14 l of ethane (c2h6) and 14 l of oxygen (...
Questions

Mathematics, 07.05.2021 05:10



Mathematics, 07.05.2021 05:10

Advanced Placement (AP), 07.05.2021 05:10


Mathematics, 07.05.2021 05:10







History, 07.05.2021 05:20




Mathematics, 07.05.2021 05:20


Mathematics, 07.05.2021 05:20