
Chemistry, 07.07.2019 13:30 lazavionadams81
The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3si(> 4cr(l) + 3sio2 (s) the reaction is begun with 167.00 g of si and 146.00 g of cr2o3. how many grams of the excess reactant are left after the reaction is complete? i found which one was the l. r but i can't figure out how to find the excess amount of the e. r. will be greatly

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3...
Questions

Biology, 03.12.2020 01:50

History, 03.12.2020 01:50

English, 03.12.2020 01:50


Biology, 03.12.2020 01:50

English, 03.12.2020 01:50


Mathematics, 03.12.2020 01:50




Mathematics, 03.12.2020 01:50

Mathematics, 03.12.2020 01:50


Mathematics, 03.12.2020 01:50

Mathematics, 03.12.2020 01:50

Mathematics, 03.12.2020 01:50

Mathematics, 03.12.2020 01:50


Mathematics, 03.12.2020 01:50

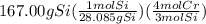
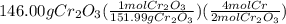
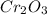 gives the less moles of the product and so it is limiting reactant and hence the excess reactant is Si.
gives the less moles of the product and so it is limiting reactant and hence the excess reactant is Si.


