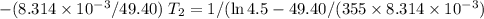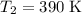Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Acertain reaction has an activation energy of 49.40 kj/mol. at what kelvin temperature will the reac...
Questions


Mathematics, 26.07.2021 22:20

Biology, 26.07.2021 22:20







Mathematics, 26.07.2021 22:20

Mathematics, 26.07.2021 22:20

Mathematics, 26.07.2021 22:20

Mathematics, 26.07.2021 22:20

Mathematics, 26.07.2021 22:20


Health, 26.07.2021 22:20



Mathematics, 26.07.2021 22:20

Mathematics, 26.07.2021 22:20

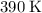
 if the concentration of all reactants in its rate-determining step is held constant. The rate "constant" is dependent on both the temperature and the activation energy of this particular reaction, as seen in the Arrhenius equation:
if the concentration of all reactants in its rate-determining step is held constant. The rate "constant" is dependent on both the temperature and the activation energy of this particular reaction, as seen in the Arrhenius equation: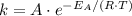
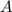 the frequency factor constant unique to this reaction
the frequency factor constant unique to this reaction the base of natural logarithms, and
the base of natural logarithms, and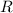 the ideal gas constant.
the ideal gas constant.
 , such that
, such that 
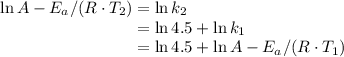
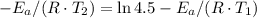
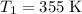 and activation energy
and activation energy 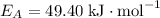 - assumed to be independent of temperature variations,
- assumed to be independent of temperature variations,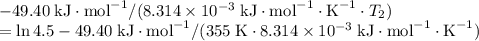
 :
: