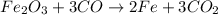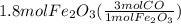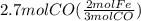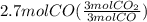Read the chemical equation. fe2o3 + co → fe + co2 if 1.8 moles of fe2o3 react with 2.7 moles of co, how many moles of each product are formed? 5.4 moles fe and 1.8 moles co2 2.7 moles fe and 0.9 moles co2 3.6 moles fe and 5.4 moles co2 1.8 moles fe and 2.7 moles co2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
Read the chemical equation. fe2o3 + co → fe + co2 if 1.8 moles of fe2o3 react with 2.7 moles of co,...
Questions



History, 18.12.2020 03:50

Mathematics, 18.12.2020 03:50

Mathematics, 18.12.2020 03:50


Mathematics, 18.12.2020 03:50


Mathematics, 18.12.2020 03:50

Mathematics, 18.12.2020 03:50

Chemistry, 18.12.2020 03:50



Mathematics, 18.12.2020 03:50


English, 18.12.2020 03:50

Social Studies, 18.12.2020 03:50


Mathematics, 18.12.2020 03:50

Mathematics, 18.12.2020 03:50

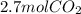 .
.