
Chemistry, 07.07.2019 03:00 TheHomieJaay3092
When aluminum is placed in concentrated hydrochloric acid, hydrogen gas is produced. 2al(s)+6hcl(aq)-> 2alcl3(aq) +3h2(g) what mass of al(s) is required to produce 513.0 ml of h2(g) at stp?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
When aluminum is placed in concentrated hydrochloric acid, hydrogen gas is produced. 2al(s)+6hcl(aq)...
Questions


Chemistry, 30.07.2019 21:00







Computers and Technology, 30.07.2019 21:00

Social Studies, 30.07.2019 21:00


History, 30.07.2019 21:00


Mathematics, 30.07.2019 21:00

Mathematics, 30.07.2019 21:00



Mathematics, 30.07.2019 21:00

Health, 30.07.2019 21:00

History, 30.07.2019 21:00

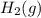 at STP = 513 ml = 0.513 L ( 1 L = 1000 ml )
at STP = 513 ml = 0.513 L ( 1 L = 1000 ml )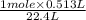 moles of
moles of 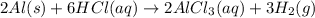
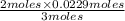 = 0.0153 moles
= 0.0153 moles

