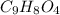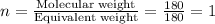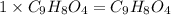Chemistry, 07.07.2019 02:30 jesh0975556
Aspirin has a molar mass of 180g/mol. if the empirical formula is c9h8o4, what is the molecular formula of aspirin ? fill in the blanks for the subscripts of the formula below. you have to have a whole number subscript for each blank even if it is a 1. c__h__o__


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
Aspirin has a molar mass of 180g/mol. if the empirical formula is c9h8o4, what is the molecular form...
Questions







Social Studies, 17.04.2020 03:51


English, 17.04.2020 03:51




Mathematics, 17.04.2020 03:51

Mathematics, 17.04.2020 03:51

Chemistry, 17.04.2020 03:51

Physics, 17.04.2020 03:51



Mathematics, 17.04.2020 03:51