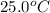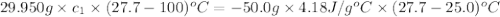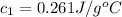Chemistry, 07.07.2019 02:00 592400014353
A29.950 g sample of an unknown metal is heated to 100.0 °c. the metal is then poured into 50.0 g of water in an insulated coffee cup calorimeter. the temperature of water rises from 25.0 ºc to 27.7 ºc. what is the specific heat of the metal?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
You know the right answer?
A29.950 g sample of an unknown metal is heated to 100.0 °c. the metal is then poured into 50.0 g of...
Questions


Mathematics, 29.07.2020 06:01






Mathematics, 29.07.2020 06:01






Social Studies, 29.07.2020 06:01






Mathematics, 29.07.2020 06:01

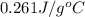
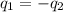
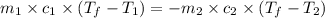
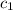 = specific heat of unknown metal = ?
= specific heat of unknown metal = ?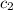 = specific heat of water =
= specific heat of water = 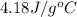
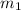 = mass of unknown metal = 29.950 g
= mass of unknown metal = 29.950 g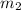 = mass of water = 50.0 g
= mass of water = 50.0 g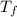 = final temperature of water =
= final temperature of water = 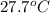
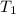 = initial temperature of unknown metal =
= initial temperature of unknown metal = 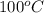
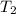 = initial temperature of water =
= initial temperature of water =