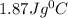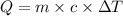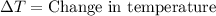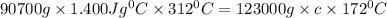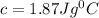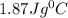Upon landing, the 90.7 kg carbon fiber brakes of an airliner heat up 312∘c, producing heat. as the brakes start to cool back to their initial temperature, the heat is absorbed by the 123 kg rubber tires. assuming that all of the heat is transferred from the brakes to the tires, what is the specific heat of the tires if their temperature rises 172∘c? round your answer to two decimal places. use 1.400jg∘c for the specific heat of carbon fiber.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
Upon landing, the 90.7 kg carbon fiber brakes of an airliner heat up 312∘c, producing heat. as the b...
Questions

SAT, 07.12.2021 03:40

SAT, 07.12.2021 03:40

Mathematics, 07.12.2021 03:40

Mathematics, 07.12.2021 03:40


Mathematics, 07.12.2021 03:40

Mathematics, 07.12.2021 03:40


Geography, 07.12.2021 03:40

SAT, 07.12.2021 03:40

SAT, 07.12.2021 03:40



Biology, 07.12.2021 03:40

Biology, 07.12.2021 03:40

Social Studies, 07.12.2021 03:40

Mathematics, 07.12.2021 03:40


Mathematics, 07.12.2021 03:40

Mathematics, 07.12.2021 03:40