
At 2000 ∘c the equilibrium constant for the reaction 2no(g)⇌n2(g)+o2(g) is kc=2.4×103. you may want to reference (pages 641 - 644) section 15.6 while completing this problem. part a if the initial concentration of no is 0.175 m, what is the equilibrium concentration of no? g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
At 2000 ∘c the equilibrium constant for the reaction 2no(g)⇌n2(g)+o2(g) is kc=2.4×103. you may want...
Questions

English, 03.12.2020 20:10


Physics, 03.12.2020 20:10


Arts, 03.12.2020 20:10



Arts, 03.12.2020 20:10


Social Studies, 03.12.2020 20:10


Mathematics, 03.12.2020 20:10


Mathematics, 03.12.2020 20:10


Mathematics, 03.12.2020 20:10

Mathematics, 03.12.2020 20:10



 .
.
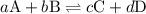
![{K_{\text{c}}}=\dfrac{{{{\left[ {\text{C}} \right]}^c}{{\left[ {\text{D}} \right]}^d}}}{{{{\left[ {\text{A}} \right]}^a}{{\left[ {\text{B}} \right]}^b}}}](/tpl/images/0058/9264/8d53d.png)
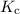 is the equilibrium constant.
is the equilibrium constant.
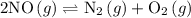
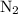 and
and  become x at equilibrium.
become x at equilibrium.
![{K_{\text{c}}}=\dfrac{{\left[ {{{\text{N}}_2}} \right]\left[{{{\text{O}}_2}} \right]}}{{{{\left[ {{\text{NO}}} \right]}^2}}}](/tpl/images/0058/9264/f4ed4.png) …… (1)
…… (1)  for
for 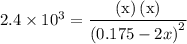 …… (2)
…… (2)


![\begin{aligned}\left[ {{\text{NO}}} \right]&= 0.175 - 2\left( {0.0866} \right)\\&= {\text{ 0}}{\text{.0018 M}}\\\end{aligned}](/tpl/images/0058/9264/23268.png)


