
If 23.7 g of al(oh)3(s) are mixed with 29.5 g of h2so4(s) and the reaction is run, answer the following questions: (a) what is the limiting reagent? (b) assuming no side reactions, how much h2o(l) can be produced under these conditions? (c) if 2.21 g of h2o(g) are produced in the lab under these conditions, what is the percent yield? (d) how many grams of the reactant in excess remains at the end of the experiment? data: atomic mass al = 26.98, h = 1.008, o = 16.00, s = 32.07,

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
If 23.7 g of al(oh)3(s) are mixed with 29.5 g of h2so4(s) and the reaction is run, answer the follow...
Questions







Biology, 31.08.2019 10:00


English, 31.08.2019 10:00



Mathematics, 31.08.2019 10:00

Biology, 31.08.2019 10:00

Physics, 31.08.2019 10:00

History, 31.08.2019 10:00

History, 31.08.2019 10:00

Biology, 31.08.2019 10:00

English, 31.08.2019 10:00


Mathematics, 31.08.2019 10:00

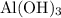 can behave as a base and neutralize sulfuric acid
can behave as a base and neutralize sulfuric acid 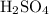 as in the following equation:
as in the following equation: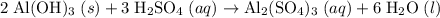 (Balanced)
(Balanced)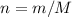 . Thus the ratio between the number of moles of the two reactants available:
. Thus the ratio between the number of moles of the two reactants available:![n(\text{Al}(\text{OH})_3, \text{supplied}) / n(\text{H}_2\text{SO}_4, \text{supplied})\\= [m(\text{Al}(\text{OH})_3)/ M(\text{Al}(\text{OH})_3)] / [n(\text{H}_2\text{SO}_4) / M(\text{H}_2\text{SO}_4)]\\= [23.7 / (26.98 + 3 \times(16.00 + 1.008))]/[29.5 / (2 \times 1.008 + 32.07 + 4 \times 16.00)]\\\approx 1.01](/tpl/images/0058/7818/2e46d.png)
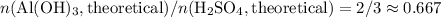
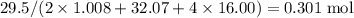 of sulfuric acid is supplied in this reaction as the limiting reagent.
of sulfuric acid is supplied in this reaction as the limiting reagent.  moles of water molecules are produced for every
moles of water molecules are produced for every  moles of sulfuric acid consumed. The reaction would thus give rise to
moles of sulfuric acid consumed. The reaction would thus give rise to 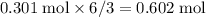 of water molecules, which have a mass of
of water molecules, which have a mass of 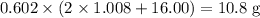 .
.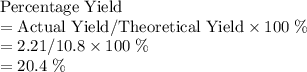
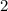 moles of aluminum hydroxide
moles of aluminum hydroxide 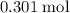 of sulfuric acid is initially available as previously stated such that
of sulfuric acid is initially available as previously stated such that 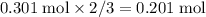 , or
, or 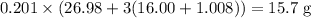 , of
, of 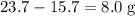 of
of 

