
What does the pauli exclusion principle state?
a) electrons will be added to an atom at the lowest possible level or sublevel.
b) an orbital can contain two electrons only if all other orbitals at that sublevel are empty.
c) an orbital can contain two electrons only if all other orbitals at that sublevel contain at least one electron.
d) two electrons occupy the same orbital only if they have opposite spins.
e) an orbital can contain two electrons only if all other orbitals at that sublevel are empty.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
What does the pauli exclusion principle state?
a) electrons will be added to an atom a...
a) electrons will be added to an atom a...
Questions

English, 26.10.2020 19:10

Mathematics, 26.10.2020 19:10

History, 26.10.2020 19:10

Chemistry, 26.10.2020 19:10

Chemistry, 26.10.2020 19:10










Chemistry, 26.10.2020 19:10


Mathematics, 26.10.2020 19:10


Mathematics, 26.10.2020 19:10

Mathematics, 26.10.2020 19:10

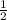 or
or 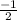 .
.

