
Chemistry, 03.12.2019 02:31 Nessakona1
Question 1 (true/false worth 4 points) (03.06 lc) an instantaneous dipole occurs when a molecule's moving electrons are briefly more concentrated in one place than another, causing the molecule to become temporarily polarized.
true false
question 2(multiple choice worth 4 points) (03.06 lc) what type of intermolecular force occurs between all substances?
covalent bonding
hydrogen bonding
ion-dipole force
london dispersion force
question 3(multiple choice worth 4 points) (03.06 mc) when comparing h2, nh3, o2, and ch4, which of the following statements is correct?
ch4 has the highest boiling point because it experiences dipole-dipole forces.
h2 has the strongest intermolecular forces because it has the lowest mass. nh3 has the highest boiling point because it experiences hydrogen bonding.
o2 has the strongest intermolecular force because it experiences london dispersion forces.
question 4(multiple choice worth 4 points) (03.06 mc)
the boiling points of diatomic halogens are compared in the table.
boiling points of diatomic halogens molecule boiling point
f2 −188 °c
cl2 −34 °c
br2 59 °c
i2 184 °c
which of the following statements best explains the trends in boiling points?
the atomic size increases down the group, and this decreases the strength of the intermolecular forces.
the total number of electrons decreases down the group, and this decreases the strength of the intermolecular forces.
the total number of electrons increases down the group, and this increases the strength of the intermolecular forces.
the chances of forming a permanent dipole increase down the group and this increases the strength of the intermolecular forces.
question 5(multiple choice worth 4 points) (03.06 mc) what is the strongest intermolecular force that occurs between molecules of co2?
dipole-dipole
induced dipoles
ionic bonding
london dispersion
asap 30 mark brainliest

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
Question 1 (true/false worth 4 points) (03.06 lc) an instantaneous dipole occurs when a molecule's m...
Questions









Mathematics, 07.07.2019 06:30


Spanish, 07.07.2019 06:30

History, 07.07.2019 06:30



Social Studies, 07.07.2019 06:30

Physics, 07.07.2019 06:30


Business, 07.07.2019 06:30

Mathematics, 07.07.2019 06:30

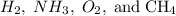 which of the following statements is correct?
which of the following statements is correct?
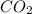 ?
?


