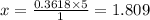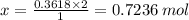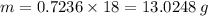Chemistry, 06.07.2019 06:30 jscout2468
You are given the balanced chemical equation: c4h4 + 5o2 4co2 + 2h2o. if 0.3618 moles of c4h4 are allowed to react with 1.818 moles of o2, and this is the only reaction which occurs, what is the maximum mass of water that could be produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
You know the right answer?
You are given the balanced chemical equation: c4h4 + 5o2 4co2 + 2h2o. if 0.3618 moles of c4h4 are a...
Questions

History, 03.12.2020 01:00


History, 03.12.2020 01:00




Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00



English, 03.12.2020 01:00

History, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

Biology, 03.12.2020 01:00


Social Studies, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00