
Which of the following shows a chemical reaction in which silane and oxygen are the reactants and silicon dioxide and water are the products? a. silicon dioxide + water → silane + oxygen b. silane + water → silicon dioxide + oxygen c. oxygen + silicon dioxide → silane + water d. silane + oxygen → silicon dioxide + water

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1


Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2
You know the right answer?
Which of the following shows a chemical reaction in which silane and oxygen are the reactants and si...
Questions

History, 28.09.2019 15:10





Mathematics, 28.09.2019 15:10


History, 28.09.2019 15:10

Health, 28.09.2019 15:10


Mathematics, 28.09.2019 15:10

Mathematics, 28.09.2019 15:10



Mathematics, 28.09.2019 15:10

History, 28.09.2019 15:10


History, 28.09.2019 15:10


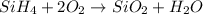
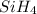 is the formula of silane. Here, both
is the formula of silane. Here, both 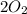 are the reactants. Whereas both
are the reactants. Whereas both 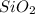 and
and 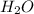 are the products.
are the products. silicon dioxide + water, represents a chemical reaction.
silicon dioxide + water, represents a chemical reaction.

