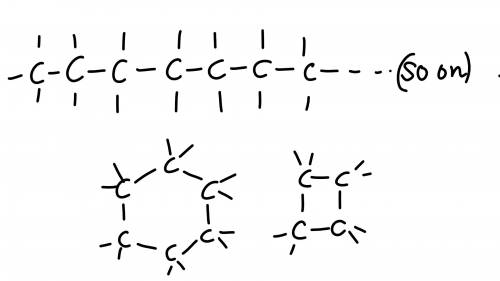Chemistry, 06.07.2019 05:30 sliverx201
25 pointsquestion how does the structure of a carbon atom enable it to form large molecules? available choices [a] each carbon atom can be stable with one, two, three, or four bonds because of how its valence electrons are arranged. [b] each carbon atom can bond with several other carbon atoms because of how many valence electrons it has. [c] each carbon atom donates its electrons to other atoms, including atoms of noble gases and halogens. [d] each carbon atom forms either double or triple bonds with surrounding hydrogen atoms. try your best, this is important, 25 points and brainliest answer if you get it right!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
25 pointsquestion how does the structure of a carbon atom enable it to form large molecules? availab...
Questions

History, 21.02.2020 19:27




Computers and Technology, 21.02.2020 19:27




Health, 21.02.2020 19:28


History, 21.02.2020 19:28

Mathematics, 21.02.2020 19:28


History, 21.02.2020 19:28

Engineering, 21.02.2020 19:28



Chemistry, 21.02.2020 19:29