
Several solutions of nickel (ii) sulfate ranging from 1.00m to 0.200 m are exposed to light in a colorimeter and their absorbance is recorded in a beer-lambert law experiment. why does a solution of nickel(ii) sulfate represent a suitable solution to be studied in this experiment, whereas similar solutions of sodium chloride do not?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
You know the right answer?
Several solutions of nickel (ii) sulfate ranging from 1.00m to 0.200 m are exposed to light in a col...
Questions






Geography, 15.04.2020 02:07


English, 15.04.2020 02:08


Biology, 15.04.2020 02:08

Mathematics, 15.04.2020 02:08

Social Studies, 15.04.2020 02:08








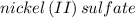 is green in color but a solution of sodium chloride is colorless that is why
is green in color but a solution of sodium chloride is colorless that is why 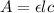
 = molar absorptivity
= molar absorptivity

