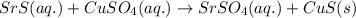Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
Aqueous strontium sulfide and aqueous copper(ii) sulfate predict the products of each of these reac...
Questions





Mathematics, 12.11.2019 02:31

Mathematics, 12.11.2019 02:31



Mathematics, 12.11.2019 02:31


Mathematics, 12.11.2019 02:31

Mathematics, 12.11.2019 02:31