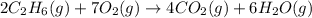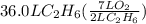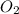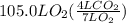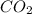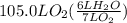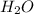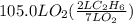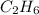Chemistry, 05.07.2019 22:00 aidendespatieshakim
How many liters of gas will be in the closed reaction flask when 36.0l of ethane (c2h6) is allowed to react with 105.0l of oxygen (under constant pressure and temperature) to form carbon dioxide gas and water vapor? assume ideal gas behavior.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 14:20
What kind of chemical reaction does the chemical equation sodium + chlorine → sodium chloride represent? a. combustion b. decomposition c. single replacement d. synthesis
Answers: 1
You know the right answer?
How many liters of gas will be in the closed reaction flask when 36.0l of ethane (c2h6) is allowed t...
Questions


Mathematics, 10.10.2019 15:30

Mathematics, 10.10.2019 15:30



Arts, 10.10.2019 15:30




Chemistry, 10.10.2019 15:30


Social Studies, 10.10.2019 15:30

Mathematics, 10.10.2019 15:30



Biology, 10.10.2019 15:30

Mathematics, 10.10.2019 15:30

Mathematics, 10.10.2019 15:30


Biology, 10.10.2019 15:30