
Chemistry, 05.07.2019 20:00 iamabouttofail
Acompound is used as a food additive. the compound has a molar mass of 176.124 grams/mole. a 692.5-gram sample undergoes decomposition, producing 283.4 grams of carbon, 31.7 grams of hydrogen, and 377.4 grams of oxygen. what is the molecular formula of the compound? a. c3h4o3 b. c4h8o4 c. c6h6o6 d. c6h8o6 e. c6h8o8

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
Acompound is used as a food additive. the compound has a molar mass of 176.124 grams/mole. a 692.5-g...
Questions


Mathematics, 16.06.2020 16:57



Business, 16.06.2020 16:57


Spanish, 16.06.2020 16:57



Mathematics, 16.06.2020 16:57


English, 16.06.2020 16:57

English, 16.06.2020 16:57

Mathematics, 16.06.2020 16:57

Mathematics, 16.06.2020 16:57


Mathematics, 16.06.2020 16:57

Geography, 16.06.2020 16:57

Mathematics, 16.06.2020 16:57

Mathematics, 16.06.2020 16:57

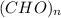 .
.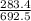 ×100 = 40.924.
×100 = 40.924.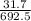 ×100 = 4.577.
×100 = 4.577.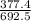 ×100 = 54.498.
×100 = 54.498.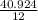 = 3.410
= 3.410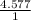 = 4.577
= 4.577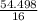 = 3.406
= 3.406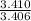 = 1,
= 1, 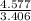 = 1 and
= 1 and 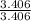 = 1
= 1![[CH_{1}O] _{n}](/tpl/images/0055/2767/aa95a.png) (where n = integer.
(where n = integer.

