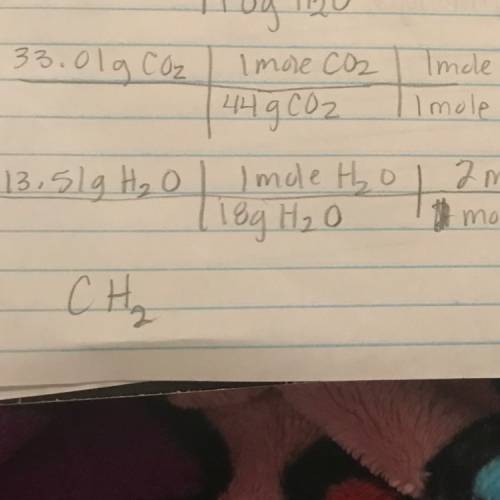Chemistry, 05.07.2019 11:30 claudiapineda860
Combustion analysis of a hydrocarbon produces 33.01 g co2 and 13.51 g h2o. calculate the empirical formula of the hydrocarbon.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 08:50
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas.caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible.relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
Combustion analysis of a hydrocarbon produces 33.01 g co2 and 13.51 g h2o. calculate the empirical f...
Questions


Biology, 01.01.2020 06:31

History, 01.01.2020 06:31


English, 01.01.2020 06:31


History, 01.01.2020 06:31

Physics, 01.01.2020 06:31

Arts, 01.01.2020 06:31



Mathematics, 01.01.2020 06:31

Social Studies, 01.01.2020 06:31






Biology, 01.01.2020 06:31