

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1


Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1
You know the right answer?
In standardizing the solution of aqueous sodium hydroxide, a chemist overshoots the end point and ad...
Questions




















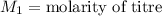 ,
, 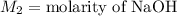
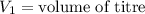 ,
, 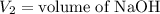
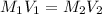
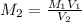 ....(1)
....(1)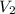 .
.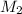 in equation (1), will get lowered , which means that the concentration of NaOH was lower than that of the actual value. Hence underestimated concentration of NaOH.
in equation (1), will get lowered , which means that the concentration of NaOH was lower than that of the actual value. Hence underestimated concentration of NaOH. 

