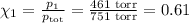Amixture of 60 mol % n-propylcyclohexane and 40 mol % n-propylbenzene is distilled through a simple distillation apparatus; assume that no fractionation occurs during the distillation. the boiling temperature is found to be 157 degrees celsius (760 torr) as the first small amount of distillate is collected. the standard vapor pressures of n-propylcyclohexane and n-propyl-benzene are known to be 769 torr and 725 torr, respectively, at 1567.3 degrees celsius. calculate the percentage of each of the two components in the first few drops of distillate.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
Amixture of 60 mol % n-propylcyclohexane and 40 mol % n-propylbenzene is distilled through a simple...
Questions

Mathematics, 11.11.2021 14:00




English, 11.11.2021 14:00

Computers and Technology, 11.11.2021 14:00

English, 11.11.2021 14:00


Mathematics, 11.11.2021 14:00

Biology, 11.11.2021 14:00

Medicine, 11.11.2021 14:00

Mathematics, 11.11.2021 14:00


History, 11.11.2021 14:00





Engineering, 11.11.2021 14:00

Social Studies, 11.11.2021 14:00

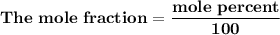
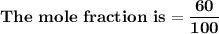
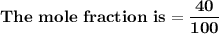
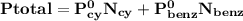
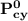 = n-propylcyclohexane vapor pressure
= n-propylcyclohexane vapor pressure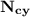 = n-propylcyclohexane mole fraction
= n-propylcyclohexane mole fraction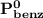 = n-propylbenzene vapor pressure
= n-propylbenzene vapor pressure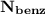 = n-propylbenzene mole fraction
= n-propylbenzene mole fraction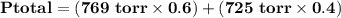
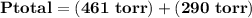

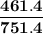
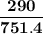
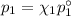 and
and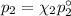
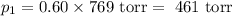
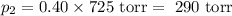
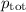 = p₁ + p₂= 461 torr + 290 torr = 751 torr
= p₁ + p₂= 461 torr + 290 torr = 751 torr