
Chemistry, 05.07.2019 11:00 Ashley606hernandez
An 8.65 g sample of an unknown group 2a metal hydroxide is dissolved in 85.0 ml of water and titrated with 2.50 m hcl(aq). if it takes 56.9 ml of the acid to reach the end point of the titration (a) what is the molar mass of the metal hydroxide? (b) which of the following is in the metal hydroxide: ca2+, sr2+, ba2+?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
An 8.65 g sample of an unknown group 2a metal hydroxide is dissolved in 85.0 ml of water and titrate...
Questions

Computers and Technology, 19.08.2020 23:01


Computers and Technology, 19.08.2020 23:01

English, 19.08.2020 23:01




English, 19.08.2020 23:01

English, 19.08.2020 23:01


Computers and Technology, 19.08.2020 23:01



Mathematics, 19.08.2020 23:01

Mathematics, 19.08.2020 23:01

Computers and Technology, 19.08.2020 23:01


History, 19.08.2020 23:01

Mathematics, 19.08.2020 23:01

History, 19.08.2020 23:01

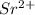 .
.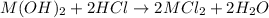
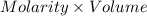
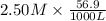
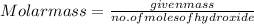
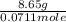
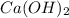 = (40.078 + 34) g/mol = 74.093 g/mol
= (40.078 + 34) g/mol = 74.093 g/mol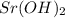 = (87.66 + 34) g/mol = 121.66 g/mol
= (87.66 + 34) g/mol = 121.66 g/mol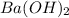 = (137.32 + 34) g/mol = 171.32 g/mol
= (137.32 + 34) g/mol = 171.32 g/mol

