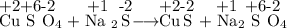Chemistry, 05.07.2019 09:00 DeGeneral8468
Did an oxidation-reduction reaction occur in the reaction between copper sulfate and sodium sulfide? cuso4 + na2s → cus + na2so4 a. this is a redox reaction because copper gained electrons. b. this is a redox reaction because sodium lost electrons. c. this is a redox reaction because sulfur gained electrons. d. this is not a redox reaction because no electrons were transferred. e. this is not a redox reaction because oxygen did not gain electrons. in which reaction is precipitation occurring? a. mgcl2(aq) + cuso4(aq) → cucl2(aq) + mgso4(aq) b. cdso4(aq) + k2s(aq) → cds(s) + k2so4(aq) c. naoh(aq) + nh4cl(aq) → nacl(aq) + nh4oh(aq) d. k2so4(aq) + naoh(aq) → k2oh(aq) + naso4(aq) e. hno3(aq) + koh(aq) → kno3(aq) + h2o(l)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
Did an oxidation-reduction reaction occur in the reaction between copper sulfate and sodium sulfide?...
Questions


History, 25.05.2021 22:10

Mathematics, 25.05.2021 22:10

Business, 25.05.2021 22:10



Mathematics, 25.05.2021 22:10




Computers and Technology, 25.05.2021 22:10

Mathematics, 25.05.2021 22:10

Physics, 25.05.2021 22:10



Chemistry, 25.05.2021 22:10