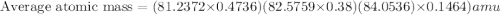Chemistry, 05.07.2019 08:30 aduncan3426
The element loncapium has three isotopes. the first lc-82 has a mass of 81.2372 amu and makes up 47.36% of a standard sample. the second isotope, lc-83, has a mass of 82.5759 amu and makes up 38% of a standard sample. the third isotope, lc-85 has a mass of 84.0536 amu and makes up the remainder of the sample. what is the average atomic mass of this element?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
The element loncapium has three isotopes. the first lc-82 has a mass of 81.2372 amu and makes up 47....
Questions

English, 07.10.2019 15:00

Social Studies, 07.10.2019 15:00




Social Studies, 07.10.2019 15:00


English, 07.10.2019 15:00



Business, 07.10.2019 15:00


English, 07.10.2019 15:00


Mathematics, 07.10.2019 15:00

Chemistry, 07.10.2019 15:00

History, 07.10.2019 15:00


Chemistry, 07.10.2019 15:00

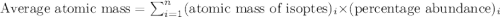 ....(1)
....(1)