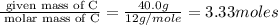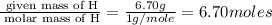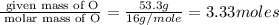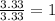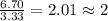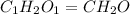Chemistry, 05.07.2019 08:30 hooplikenari
Acompound is 40.0% c, 6.70% h, and 53.3% o by mass. assume that we have a 100.-g sample of this compound. part a what are the subscripts in the empirical formula of this compound? enter the subscripts for c, h, and o, respectively, separated by commas (e. g., 5,6,7).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
Acompound is 40.0% c, 6.70% h, and 53.3% o by mass. assume that we have a 100.-g sample of this comp...
Questions



Arts, 27.09.2020 17:01





Biology, 27.09.2020 17:01

Mathematics, 27.09.2020 17:01

Mathematics, 27.09.2020 17:01

Mathematics, 27.09.2020 17:01

Mathematics, 27.09.2020 17:01


Mathematics, 27.09.2020 17:01

Mathematics, 27.09.2020 17:01


Physics, 27.09.2020 17:01

Mathematics, 27.09.2020 17:01


Mathematics, 27.09.2020 17:01