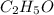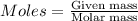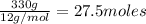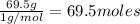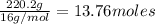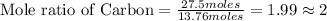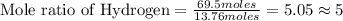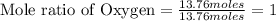Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 23.06.2019 18:00
Amolecule is a(n) - a. element that physically combines with another element. b. particle composed of two or more atoms bonded together covalently. c. element that isn't bonded to another element.
Answers: 1

Chemistry, 23.06.2019 23:30
Which of the following changes has a decrease in entropy? (3 points) n2o4 (g) yields 2no2 (g) c6h6 (l) yields c6h6 (g) 2liclo3 (s) yields 3o2 (g) + 2licl (s) 3fe (s) + 2o2 (g) yields fe3o4 (g)
Answers: 3

Chemistry, 24.06.2019 03:30
Familiar solutions can have a wide range of ph levels. according to the chart, which is the strongest acid, based on its ph level? lemon juice has approximately how many more times the h+ ions than tomato juice? which has the highest concentration of h+ ions: baking soda, ammonia, or bleach?
Answers: 3
You know the right answer?
Asample of a compound is decomposed in the laboratory and produces 330 g carbon, 69.5 g hydrogen, an...
Questions


Health, 16.10.2019 02:00








Social Studies, 16.10.2019 02:00

Mathematics, 16.10.2019 02:00