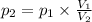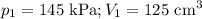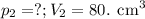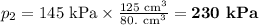Chemistry, 05.07.2019 02:00 cairolove228
At 25°c, gas in a rigid cylinder with a movable piston has a volume of 145 ml and a pressure of 125 kpa. then the gas is compressed to a volume of 80. ml. what is the new pressure of the gas if the temperature is held at 25°c? (1) 69 kpa (3) 160 kpa (2) 93 kpa (4) 230 kpa the first person didn't make sense as p1v1 = p2v2 isnt the same thing as p1v1/v2 that literally doesn't make sense, so could someone me !

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
At 25°c, gas in a rigid cylinder with a movable piston has a volume of 145 ml and a pressure of 125...
Questions



Computers and Technology, 23.03.2020 20:00

Mathematics, 23.03.2020 20:00


Mathematics, 23.03.2020 20:00





Mathematics, 23.03.2020 20:00


Computers and Technology, 23.03.2020 20:00





Physics, 23.03.2020 20:00

Mathematics, 23.03.2020 20:00

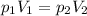
 .
.