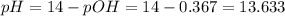Chemistry, 04.07.2019 23:00 smartgirl2092
What is ph when 4.0 ml of 2.0 m barium hydroxide is added to 10.0 ml of 1.00 m nitric acid? 13.63 0.37 0.85 13.15

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
What is ph when 4.0 ml of 2.0 m barium hydroxide is added to 10.0 ml of 1.00 m nitric acid? 13.63...
Questions

Mathematics, 13.10.2020 16:01

Physics, 13.10.2020 16:01


Mathematics, 13.10.2020 16:01

Mathematics, 13.10.2020 16:01


English, 13.10.2020 16:01

Social Studies, 13.10.2020 16:01

Mathematics, 13.10.2020 16:01

Mathematics, 13.10.2020 16:01

Mathematics, 13.10.2020 16:01





Mathematics, 13.10.2020 16:01

Mathematics, 13.10.2020 16:01


Computers and Technology, 13.10.2020 16:01

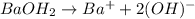
 (volume in liters) ...(1)(1L=1000mL)
(volume in liters) ...(1)(1L=1000mL)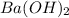 in 2.0 M solution
in 2.0 M solution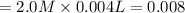 moles
moles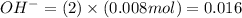 moles
moles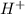 ions in nitric acid
ions in nitric acid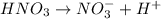
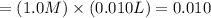 moles
moles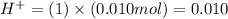 moles
moles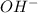
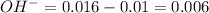 moles
moles![[OH^-]](/tpl/images/0051/9158/b2910.png) resulting solution
resulting solution![[OH^-]=\frac{0.006}{0.004L+0.010 L}=0.4285 M](/tpl/images/0051/9158/7313c.png)
![pOH=-log[OH^-]=-log(0.4285)=0.367](/tpl/images/0051/9158/4b947.png)