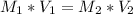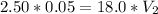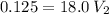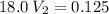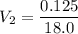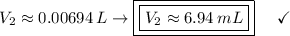In a school’s laboratory, students require 50.0 ml of 2.50 m h2so4 for an experiment, but the only available stock solution of the acid has a concentration of 18.0 m. what volume of the stock solution would they use to make the required solution? use mc018-1.jpg.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 14:00
If you fill your car tire to a pressure of 32 psi (pounds per square inch) on a hot summer day when the temperature is 35°c (95°f), what is the pressure (in psi) on a cold winter day when the temperature is -15°c (5°f)? assume no gas leaks out between measurements and the volume of the tire does not change.
Answers: 1

Chemistry, 23.06.2019 14:30
When does phenolphthalein turn pink? in the presence of a base in the presence of an acid when it is in a neutral solution when it is reacting with a metal
Answers: 1

Chemistry, 23.06.2019 15:00
The specific heat of a certain type of cooking oil is 1.75 cal/ (g c) how much heat energy is needed to raise the temperature of 2.67 kg of this oil from 23 c to 191 c
Answers: 1
You know the right answer?
In a school’s laboratory, students require 50.0 ml of 2.50 m h2so4 for an experiment, but the only a...
Questions



Mathematics, 03.05.2020 13:52

Mathematics, 03.05.2020 13:52

Mathematics, 03.05.2020 13:52

Mathematics, 03.05.2020 13:52

Mathematics, 03.05.2020 13:52


Mathematics, 03.05.2020 13:52





Mathematics, 03.05.2020 13:52




History, 03.05.2020 13:52

Mathematics, 03.05.2020 13:52