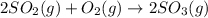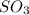How would adding the catalyst nitrogen monoxide (no) affect this reaction? 2so2(g) + o2(g) → 2so3(g) a. no increases the rate at which so3 molecules are formed. b. no reacts with so3 to produce more so2 molecules. c. no decreases collisions between the so2 and o2 molecules. d. no increases the concentration of the so2 and o2 molecules. e. no increases the activation energy of the so2 and o2 molecules.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 11:10
Why would a doctor most likely restrict a patient's contact with other people while the patient receives internal radiation
Answers: 1
You know the right answer?
How would adding the catalyst nitrogen monoxide (no) affect this reaction? 2so2(g) + o2(g) → 2so3(g...
Questions


English, 25.03.2021 17:50




Mathematics, 25.03.2021 17:50



Mathematics, 25.03.2021 17:50




Geography, 25.03.2021 17:50



Mathematics, 25.03.2021 17:50

History, 25.03.2021 17:50

English, 25.03.2021 17:50