
Chemistry, 04.07.2019 20:30 kingje1477
Calculate the standard enthalpy of combustion. the standard enthalpy of formation of sucrose is - 2226.1kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 14:00
How many moles of oxygens atoms are present in 5.00 mol of diphosphorus of fe2(so4)3
Answers: 2
You know the right answer?
Calculate the standard enthalpy of combustion. the standard enthalpy of formation of sucrose is - 22...
Questions


English, 30.10.2020 04:00


History, 30.10.2020 04:00


Mathematics, 30.10.2020 04:00


Mathematics, 30.10.2020 04:00

Biology, 30.10.2020 04:00


Social Studies, 30.10.2020 04:00

Biology, 30.10.2020 04:00


Mathematics, 30.10.2020 04:00


History, 30.10.2020 04:00


Mathematics, 30.10.2020 04:00


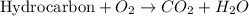
![\Delta H^o_{rxn}=\sum [n\times \Delta H_f^o_{(product)}]-\sum [n\times \Delta H_f^o_{(reactant)]}](/tpl/images/0051/4711/84379.png)
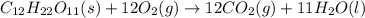
![\Delta H^o_{rxn}=[(12\times \Delta H_f^o_{(CO_2)})+(11\times \Delta H_f^o_{(H_2O)})]-[(1\times \Delta H_f^o_{(C_{12}H_{22}O_{11})})+(12\times \Delta H_f^o_{(O_2)})]](/tpl/images/0051/4711/fac1e.png)
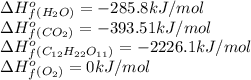
![\Delta H^o_{rxn}=[(12\times (-393.51))+(11\times (-285.8))]-[(1\times (-2226.1))+(12\times (0))]\\\\\Delta H^o_{rxn}=-5636.52kJ](/tpl/images/0051/4711/5e149.png)


