
Chemistry, 04.07.2019 14:30 jcutler2751
When a highly reactive metal, such as lithium (li), is mixed with a highly reactive nonmetal, such as chlorine (cl), they will most likely combine to form lithium chloride (licl). based on the trends of the periodic table, which other element is likely to combine with lithium? a. neon (ne) b. fluorine (f) c. silver (ag) d. calcium (ca)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1
You know the right answer?
When a highly reactive metal, such as lithium (li), is mixed with a highly reactive nonmetal, such a...
Questions


History, 18.09.2019 03:30

Mathematics, 18.09.2019 03:30

History, 18.09.2019 03:30

History, 18.09.2019 03:30

Mathematics, 18.09.2019 03:30


Mathematics, 18.09.2019 03:30



Social Studies, 18.09.2019 03:30

Biology, 18.09.2019 03:30


Mathematics, 18.09.2019 03:30



Mathematics, 18.09.2019 03:30

Biology, 18.09.2019 03:30


History, 18.09.2019 03:30

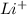 ion.
ion.

