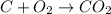Chemistry, 04.07.2019 09:30 smithmalyk4
View the diagram below describe what happened in the diagram. the molecules on both sides of the chemical reaction are the same, because they are both mixtures. bonds were broken on the reactants and new bonds were formed on the products. the molecules expanded and cooled. bonds were broken on the reactants and no new bonds were formed on the products.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
View the diagram below describe what happened in the diagram. the molecules on both sides of the che...
Questions

History, 27.05.2021 02:10


Mathematics, 27.05.2021 02:10



Biology, 27.05.2021 02:20

Mathematics, 27.05.2021 02:20

Chemistry, 27.05.2021 02:20

Mathematics, 27.05.2021 02:20







Mathematics, 27.05.2021 02:20

Mathematics, 27.05.2021 02:20


Mathematics, 27.05.2021 02:20

Mathematics, 27.05.2021 02:20