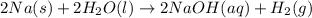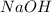Chemistry, 04.07.2019 07:00 Robinlynn228
Assign an oxidation number to each atom in the products. 2na(s)+2h2o(l)→2naoh(aq)+h2(g) assign an oxidization number for: na , o , h (in naoh) , h (in h2) i'm not sure how to assign oxidization numbers, if someone could explain it to me that'd be great! i have an exam that involves oxidization numbers coming up, and i'm completely clueless on them! you in advance!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
Assign an oxidation number to each atom in the products. 2na(s)+2h2o(l)→2naoh(aq)+h2(g) assign an ox...
Questions

History, 03.11.2020 23:30

History, 03.11.2020 23:30


Social Studies, 03.11.2020 23:30




English, 03.11.2020 23:30

English, 03.11.2020 23:30

Mathematics, 03.11.2020 23:30

Mathematics, 03.11.2020 23:30

Mathematics, 03.11.2020 23:30

Biology, 03.11.2020 23:30

Mathematics, 03.11.2020 23:30


Mathematics, 03.11.2020 23:30

English, 03.11.2020 23:30

Mathematics, 03.11.2020 23:30

Mathematics, 03.11.2020 23:30

Biology, 03.11.2020 23:30

 is zero.
is zero.