
Chemistry, 04.07.2019 03:00 princessgabbee8452
Helium has two valence electrons, and these electrons sre located in the 1s subshell. without using the periodic table, in which group and period is helium located? a .group 8a, period 1 b. group 6a, period 3 c. group 3a, period 3 d. group 3b, period 6

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2
You know the right answer?
Helium has two valence electrons, and these electrons sre located in the 1s subshell. without using...
Questions

Social Studies, 07.07.2019 06:00


History, 07.07.2019 06:00



English, 07.07.2019 06:00



Physics, 07.07.2019 06:00



Biology, 07.07.2019 06:00


Social Studies, 07.07.2019 06:00

Business, 07.07.2019 06:00


Mathematics, 07.07.2019 06:00



English, 07.07.2019 06:00

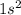 . As here n = 1 this means that helium element belongs to period 1.
. As here n = 1 this means that helium element belongs to period 1.

