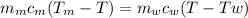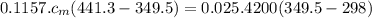Ahot lump of 115.7 g of an unknown substance initially at 168.3°c is placed in 25.0 ml of water initially at 25.0°c and allowed to reach thermal equilibrium. the final temperature of the system is 76.5°c. what is the identity of the unknown substance? assume no heat is lost to the surroundings

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
Ahot lump of 115.7 g of an unknown substance initially at 168.3°c is placed in 25.0 ml of water init...
Questions

Mathematics, 21.04.2020 03:07



Mathematics, 21.04.2020 03:07






Mathematics, 21.04.2020 03:07

Mathematics, 21.04.2020 03:07


Physics, 21.04.2020 03:07

Mathematics, 21.04.2020 03:07





Mathematics, 21.04.2020 03:07

Mathematics, 21.04.2020 03:07

 .
.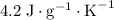 and a density of
and a density of 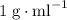 . 25.0 milliliters of water thus has a mass of 25.0 grams.
. 25.0 milliliters of water thus has a mass of 25.0 grams. 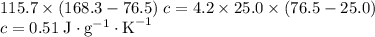
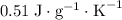 . This substance is thus probably steel.
. This substance is thus probably steel.