
Chemistry, 03.07.2019 17:30 enitramedouard12
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g); molar mass of butane 58.12g/mol calculate the mass of butane needed to produce 90.9 g of carbon dioxide. calculate the mass of butane needed to produce 90.9 g of carbon dioxide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g); molar mass of butane 58.12g/mol calculate the mass of butane ne...
Questions



English, 29.07.2020 17:01


Computers and Technology, 29.07.2020 17:01

History, 29.07.2020 17:01


Mathematics, 29.07.2020 17:01




Mathematics, 29.07.2020 17:01

Computers and Technology, 29.07.2020 17:01



Mathematics, 29.07.2020 17:01


History, 29.07.2020 18:01


Mathematics, 29.07.2020 18:01

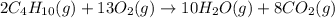
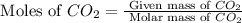
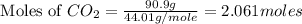
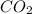 produced from 2 moles
produced from 2 moles 
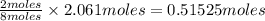 of
of 

