Chemistry, 03.07.2019 16:30 assassin42
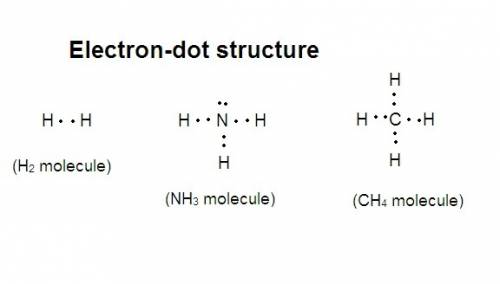
In the space provided below, draw electron-dot diagrams for the following molecules: hydrogen (h2), ammonia (nh3), and methane (ch4). remember that the dots represent the valence electrons. make sure that each atom in the molecules have 8 valence electrons, except hydrogen, which has only 2.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1
You know the right answer?
In the space provided below, draw electron-dot diagrams for the following molecules: hydrogen (h2),...
Questions

Social Studies, 25.03.2021 18:50

Chemistry, 25.03.2021 18:50

History, 25.03.2021 18:50



Mathematics, 25.03.2021 18:50

Mathematics, 25.03.2021 18:50


Mathematics, 25.03.2021 18:50

Spanish, 25.03.2021 18:50



Mathematics, 25.03.2021 18:50


History, 25.03.2021 18:50

English, 25.03.2021 18:50


Mathematics, 25.03.2021 18:50


Business, 25.03.2021 18:50