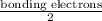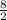Chemistry, 03.07.2019 16:30 EssenceBlocker144
Consider the lewis structure for the polyatomic oxyanion shown here, where x is an element from the third period (na−ar). by changing the overall charge, n, from 1- to 2- to 3- we get three different polyatomic ions. element x is surrounded by 4 oxygen atoms bonded to it with full octets. a)for each of these ions identify the central atom, x. arrange your answers in order increasing n. b)for each of these ions determine the formal charge of the central atom, x. arrange your answers in order increasing n. i really have no idea how to solve this. any would be greatly appreciated!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Consider the lewis structure for the polyatomic oxyanion shown here, where x is an element from the...
Questions

SAT, 08.02.2022 21:10


Social Studies, 08.02.2022 21:10


Mathematics, 08.02.2022 21:20




Chemistry, 08.02.2022 21:20

English, 08.02.2022 21:20

Mathematics, 08.02.2022 21:20



Mathematics, 08.02.2022 21:20