
After the completion of a gas forming reaction, the column of water remaining in the collection flask was measured to me 55mm high, the vapor pressure of water is 0.0313atm at 25oc, and the atmospheric pressure that day was measured as 0.950 atm, what is the partial pressure of the gas produced by the reaction? 1mmhg=13.595mm h2o and 1atm=760mmhg

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
After the completion of a gas forming reaction, the column of water remaining in the collection flas...
Questions

Computers and Technology, 25.11.2021 06:20







English, 25.11.2021 06:20

Mathematics, 25.11.2021 06:20

Social Studies, 25.11.2021 06:20

Mathematics, 25.11.2021 06:20


Physics, 25.11.2021 06:20




Mathematics, 25.11.2021 06:20



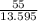 = 4.045 mm of Hg [As 1 mm of Hg is equivalent to 13.595 mm of water]
= 4.045 mm of Hg [As 1 mm of Hg is equivalent to 13.595 mm of water]

