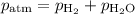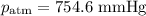Chemistry, 03.07.2019 13:30 nicky123415
Mass of metal=0.0291. volume of gas collected over water=25.67ml. temperature=24.1c. atmospheric pressure=754.6mmhg. note: metal and gas have a 1: 1 ratio in the balanced equation. the formula weight of the metal is? steps. 1) calculate partial pressure of hydrogen. 2) calculate moles of hydrogen. 3) calculate moles of metal. 4) calculate atomic mass of metal. 5) calculate relative average deviation in ppt

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3
You know the right answer?
Mass of metal=0.0291. volume of gas collected over water=25.67ml. temperature=24.1c. atmospheric pre...
Questions


Mathematics, 13.11.2020 01:00



Mathematics, 13.11.2020 01:00

Social Studies, 13.11.2020 01:00




Mathematics, 13.11.2020 01:00




Mathematics, 13.11.2020 01:00

Advanced Placement (AP), 13.11.2020 01:00

Computers and Technology, 13.11.2020 01:00




Mathematics, 13.11.2020 01:00