

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

You know the right answer?
On the basis of the reactions observed in the six test tubes, explain why the position of hydrogen c...
Questions


Business, 15.10.2019 21:40

History, 15.10.2019 21:40

Mathematics, 15.10.2019 21:40

History, 15.10.2019 21:40

Mathematics, 15.10.2019 21:40


Biology, 15.10.2019 21:40

Mathematics, 15.10.2019 21:50


Chemistry, 15.10.2019 21:50

Mathematics, 15.10.2019 21:50





Advanced Placement (AP), 15.10.2019 21:50

Social Studies, 15.10.2019 21:50

History, 15.10.2019 21:50

 ion as well as it can easily accepts an electron to complete its octet and exist as
ion as well as it can easily accepts an electron to complete its octet and exist as 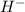 therefore it placed in group-1 (alkali metals) as well as group-17 (halogens).
therefore it placed in group-1 (alkali metals) as well as group-17 (halogens).

