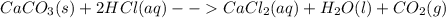The reaction between calcium carbonate (caco3) and hcl produces calcium chloride (cacl2), carbon dioxide (co2), and water (h2o).what happens when the concentration of hydrogen chloride (hcl) molecules is doubled in this reaction? caco3 + 2hcl → cacl2 + co2 + h2o when the hydrogen chloride concentration doubles, the number of collisions between the reactants (increases, decreases, remains constant) , which causes the rate of the forward reaction to (increase, decrease, remain constant)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
The reaction between calcium carbonate (caco3) and hcl produces calcium chloride (cacl2), carbon dio...
Questions

History, 18.10.2019 18:00


English, 18.10.2019 18:00


History, 18.10.2019 18:00


Physics, 18.10.2019 18:00

Health, 18.10.2019 18:00


Biology, 18.10.2019 18:00





Chemistry, 18.10.2019 18:00




Mathematics, 18.10.2019 18:00