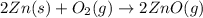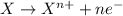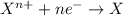Chemistry, 03.07.2019 11:30 vlactawhalm29
When zinc metal reacts with oxygen gas, 2zn(s) + o2(g) → 2zno(g), large amounts of light and heat are released. a student states that this reaction is a combustion reaction but not a redox reaction. do you agree? defend your answer by explaining whether or not it meets the requirements of each type of reaction. asap

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
When zinc metal reacts with oxygen gas, 2zn(s) + o2(g) → 2zno(g), large amounts of light and heat ar...
Questions




Mathematics, 22.09.2021 14:00


History, 22.09.2021 14:00


Mathematics, 22.09.2021 14:00


Mathematics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00


Mathematics, 22.09.2021 14:00


Social Studies, 22.09.2021 14:00


Mathematics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00

Computers and Technology, 22.09.2021 14:00