Chemistry, 03.07.2019 10:30 kaliahgrey
Use the balanced equation to work the following problem: cacl2 + 2agno3 → 2 agcl + ca(no3)2 how many grams of agcl (molar mass=143 g/mol) is formed when 1.00 gram of agno3 (molar mass=170 g/mol) reacts? 1.00 g 0.00588 g 1.19 g 0.841 g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Use the balanced equation to work the following problem: cacl2 + 2agno3 → 2 agcl + ca(no3)2 how man...
Questions





Mathematics, 15.02.2021 22:10

Chemistry, 15.02.2021 22:10






Spanish, 15.02.2021 22:10

Mathematics, 15.02.2021 22:10





Chemistry, 15.02.2021 22:10


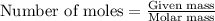


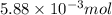 of silver nitrate will produce =
of silver nitrate will produce =  of AgCl
of AgCl