
Chemistry, 03.07.2019 02:00 brittneyrenae7338
To make use of an ionic hydrate for storing solar energy, you place 510.0 kg of sodium sulfate decahydrate on your house roof. assuming complete reaction and 100% efficiency of heat transfer, how much heat (in kj) is released to your house at night? note that sodium sulfate decahydrate will transfer 354 kj/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
To make use of an ionic hydrate for storing solar energy, you place 510.0 kg of sodium sulfate decah...
Questions


Mathematics, 28.04.2021 14:00




Mathematics, 28.04.2021 14:00


English, 28.04.2021 14:00

Mathematics, 28.04.2021 14:00




Mathematics, 28.04.2021 14:00

English, 28.04.2021 14:00

Computers and Technology, 28.04.2021 14:00

Biology, 28.04.2021 14:00

Biology, 28.04.2021 14:00


Mathematics, 28.04.2021 14:00

Chemistry, 28.04.2021 14:00

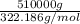 = 1582.936 mol of H₂₀Na₂O₁₄S.
= 1582.936 mol of H₂₀Na₂O₁₄S.

