Chemistry, 03.07.2019 02:00 710jonathan
Calculate the ph of the solution made by adding 0.50 mol of hobr and 0.30 mol of kobr to 1.00 l of water. the value of ka for hobr is 2.0ă—10â’9. express your answer numerically using two decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2
You know the right answer?
Calculate the ph of the solution made by adding 0.50 mol of hobr and 0.30 mol of kobr to 1.00 l of w...
Questions

Computers and Technology, 02.03.2020 21:39






Mathematics, 02.03.2020 21:39

Mathematics, 02.03.2020 21:39


Health, 02.03.2020 21:40

Health, 02.03.2020 21:40


Mathematics, 02.03.2020 21:40


Mathematics, 02.03.2020 21:40

Arts, 02.03.2020 21:40

Mathematics, 02.03.2020 21:40



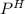 of the solution containing HOBr and KOBr.
of the solution containing HOBr and KOBr.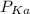 + log
+ log ![\frac{[salt]}{[acid]}](/tpl/images/0044/6662/a0d10.png)
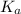 value of HOBr is 2×10⁻⁹.
value of HOBr is 2×10⁻⁹.