
Chemistry, 03.07.2019 01:30 idontknow11223344
Using iupac nomenclature, give the name of bh3 and alh3 and elaborate why that name is correct. a) boron trihydride & aluminum trihydride - both molecules combine two non-metals and form covalent bonds. b) boron hydride & aluminum hydride - both compounds combine a main group metal and a non-metal to form ionic bonds. c) boron trihydride - boron and hydrogen are both non-metals and form covalent bonds. aluminum hydride - aluminum is a main group metal and hydrogen is a non-metal and they form ionic bonds. d) boron hydride - boron is a main group metal and hydrogen is a non-metal and they form ionic bonds. aluminum trihydride - aluminum and hydrogen are both non-metals and form covalent bonds.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

You know the right answer?
Using iupac nomenclature, give the name of bh3 and alh3 and elaborate why that name is correct. a)...
Questions


Mathematics, 31.07.2019 23:20



Mathematics, 31.07.2019 23:20

Mathematics, 31.07.2019 23:20

English, 31.07.2019 23:20


Mathematics, 31.07.2019 23:20

Biology, 31.07.2019 23:20






Physics, 31.07.2019 23:20


History, 31.07.2019 23:30


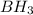 is a covalent compound because sharing of electrons takes place between hydrogen and boron. Both the elements are non-metals and hence, will form covalent bond.
is a covalent compound because sharing of electrons takes place between hydrogen and boron. Both the elements are non-metals and hence, will form covalent bond.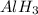 is an ionic compound because aluminium element is a metal and hydrogen element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
is an ionic compound because aluminium element is a metal and hydrogen element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.

